The Difference Between Medical Devices & In Vitro Diagnostics
A diagnostic can be a device, but not all medical devices are diagnostics. Sound confusing? Wait until we add ‘in vitro diagnostics’ to the equation.
Read on to find out the differences between the two and how they both come together to improve patients’ lives.
What is a medical diagnostic?
A medical diagnostic is a test, device, or procedure used to identify a disease, condition, or injury in a person. A diagnosis is usually achieved by analysing the signs, symptoms, and health history of an individual, ultimately guiding treatment and improving health outcomes.
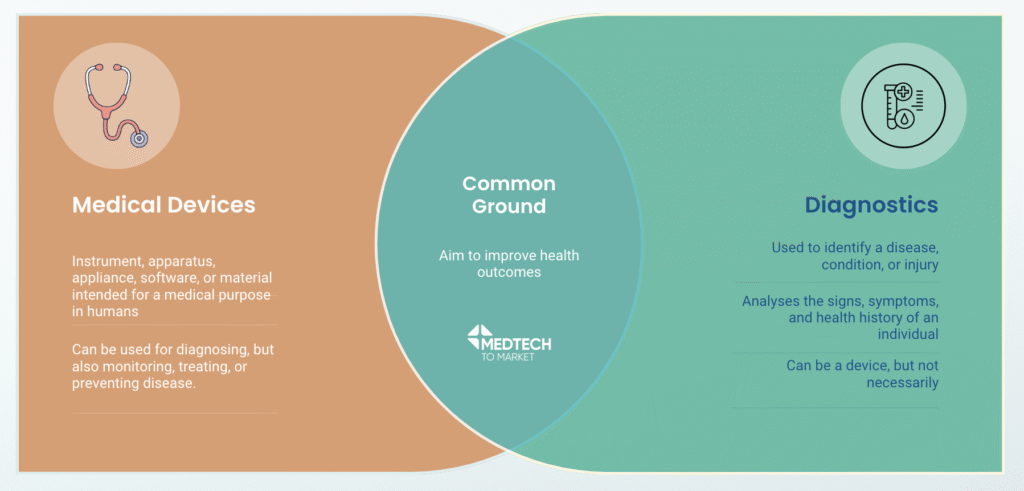
How is a diagnostic different to a medical device?
A medical device is defined as: any instrument, apparatus, appliance, software, or material intended for a medical purpose in humans, which could be diagnosing, but also monitoring, treating, or preventing disease.
Therefore, a device can be used as a diagnostic, but there are other applications, too. For example, complex equipment such as the X-ray machine (perhaps the most iconic medical device) is one that can be used as a diagnostic, while the humble bandage is also considered a medical device for its use in treatment but is not considered a diagnostic.
In vivo vs. In vitro Diagnostics (IVD)
For those of you not familiar with Latin, in vivo refers to ‘within the living’ whereas in vitro refers to ‘within the glass’
In vitro diagnostics (IVD) therefore refers to the technique of performing a given diagnostic procedure in a controlled environment outside of a living organism, e.g. samples such as tissue or blood in petri dishes or test tubes, to detect diseases or other conditions. IVD can be used to monitor a person’s general health and to aid in the treatment or prevention of diseases.
IVD assay types
Most in vitro diagnostic assays are developed based on molecular or immunodetection technologies, and include:
- Polymerase Chain Reaction (PCR)
- enzyme-linked immunosorbent assays (ELISA)
- chemiluminescent immunoassays (CLIA)
- lateral flow
- cytology
- immunohistochemistry
Famous use cases of IVD
The most well-known in vitro diagnostics are:
- Complete Blood Count (CBC):
A fundamental and frequently performed test that provides a snapshot of the blood and is often a part of routine checkups.
- Genetic Testing:
Technologies like Next-Generation Sequencing and liquid biopsies are becoming more common for diagnosing genetic disorders and certain cancers.
- Diagnostic Immunoassays:
These tests, which include things like pregnancy tests and tests for infectious diseases, are widely used in both clinical and at-home settings. (more on this below)
- Point-of-Care Testing:
Point of Care System Development (POC) is the development of fast and accurate diagnostic tools that can be used at a patient’s bedside or in the home is a major advancement, with at-home COVID-19 tests being a recent example that everyone is familiar with.

Medtechtomarket specialises in the full life cycle of in-vitro diagnostics (IVD) and diagnostic medical device development – from concept to commercial launch.
For 30+ years we’ve provided the expertise to companies across the globe to bring diagnostics to life. Accelerating innovation, overcoming regulatory hurdles, and delivering products that make a difference to patients’ lives.
